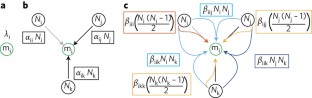
Higher-order interactions capture unexplained complexity in diverse communities
Natural communities are well known to be maintained by many complex processes. Despite this, the practical aspects of studying them often require some simplification, such as the widespread assumption that direct, additive competition captures the important details about how interactions between species impact community diversity. More complex non-additive ‘higher-order’ interactions are assumed to be negligible or absent. Notably, these assumptions are poorly supported and have major consequences for the accuracy with which patterns of natural diversity are modelled and explained. We present a mathematically simple framework for incorporating biologically meaningful complexity into models of diversity by including non-additive higher-order interactions. We further provide empirical evidence that such higher-order interactions strongly influence species’ performance in natural plant communities, with variation in seed production (as a proxy for per capita fitness) explained dramatically better when at least some higher-order interactions are considered. Our study lays the groundwork for a long-overdue shift in how species interactions are used to study the diversity of natural communities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
133,45 € per year
only 11,12 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Integrative models explain the relationships between species richness and productivity in plant communities
Article Open access 24 September 2019
The evolution of trait variance creates a tension between species diversity and functional diversity
Article Open access 09 May 2022
The balance of interaction types determines the assembly and stability of ecological communities
Article 24 February 2020
References
- Chesson, P. in Encyclopedia of Sustainability Science and Technology (ed. Meyers, R. A. ) Ch. 13, 223–256 (Springer, 2012). Google Scholar
- Allesina, S. & Levine, J. M. A competitive network theory for species diversity. Proc. Natl Acad. Sci. USA108, 5638–5642 (2011). ArticleCASGoogle Scholar
- Volterra, V. Fluctuations in the abundance of a species considered mathematically. Nature118, 558–560 (1926). ArticleGoogle Scholar
- May, R. Will a large complex system be stable? Nature238, 413–414 (1972). ArticleCASGoogle Scholar
- Thuiller, W. et al. A road map for integrating eco-evolutionary processes into biodiversity models. Ecol. Lett.16, 94–105 (2013). ArticleGoogle Scholar
- Sih, A., Englund, G. & Wooster, D. Emergent impacts of multiple predators on prey. Trends Ecol. Evol.13, 350–355 (1998). ArticleCASGoogle Scholar
- HilleRisLambers, J., Adler, P. B., Harpole, W. S., Levine, J. M. & Mayfield, M. M. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. S.43, 227–248 (2012). ArticleGoogle Scholar
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst.31, 343–366 (2000). ArticleGoogle Scholar
- Kunstler, G. et al. Plant functional traits have globally consistent effects on competition. Nature529, 204–207 (2016). ArticleCASGoogle Scholar
- Levine, J. M. & HilleRisLambers, J. The importance of niches for the maintenance of species diversity. Nature461, 254–257 (2009). ArticleCASGoogle Scholar
- Falster, D. S., FitzJohn, R. G., Brännstrom, Å., Diekmann, U. & Westoby, M. Plant: a package for modelling forest trait ecology and evolution. Methods Ecol. Evol.7, 136–146 (2016). ArticleGoogle Scholar
- Madin, J. S., Hoogenboom, M. O. & Connolly, S. R. Integrating physiological and biomechanical drivers of population growth over environmental gradients on coral reefs. J. Exp. Biol.215, 968–976 (2012). ArticleGoogle Scholar
- Connolly, S. R. & Roughgarden, J. Theory of marine communities: competition, predation, and recruitment-dependent interaction strength. Ecol. Monogr.69, 277–296 (1999). ArticleGoogle Scholar
- Smith-Gill, S. J. & Gill, D. E. Curvilinearities in the competition equations: an experiment with ranid tadpoles. Am. Nat.112, 557–570 (1978). ArticleGoogle Scholar
- Wootton, T. J. The nature and consequences of indirect effects in ecological communities. Annu. Rev. Ecol. Syst.25, 443–466 (1994). ArticleGoogle Scholar
- White, E. M., Wilson, J. C. & Clarke, A. R. Biotic indirect effects: a neglected concept in invasion biology. Divers. Distrib.12, 443–455 (2006). ArticleGoogle Scholar
- Roughgarden, J. & Diamond, J. in Community Ecology (eds Diamond, J. & Case, T. J. ) 333–343 (Harper and Row, 1986). Google Scholar
- Schoener, T. W. Some methods for calculating competition coefficients for resource-utilization spectra. Am. Nat.108, 332–340 (1974). ArticleGoogle Scholar
- Freckleton, R. P. & Watkinson, A. R. Predicting competition coefficients for plant mixtures: reciprocity, transitivity and correlations with life-history traits. Ecol. Lett.4, 348–357 (2001). ArticleGoogle Scholar
- Billick, I. & Case, T. J. Higher order interactions in ecological communities: what are they and how can they be detected? Ecology75, 1529–1543 (1994). ArticleGoogle Scholar
- Werner, E. E. & Peacor, S. D. A review of trait-mediated indirect interactions in ecological communities. Ecology84, 1083–1100 (2003). ArticleGoogle Scholar
- Abrams, P. A., Menge, B. A., Mittlebach, G. G., Spiller, D. & Yodzis, P. in Food Webs: Integration of Patterns and Dynamics (eds Polis, G. & Winemiller, K. ) 371–395 (Chapman and Hall, 1996). BookGoogle Scholar
- Peacor, S. D. & Werner, E. E. The contribution of trait-mediated indirect effects to the net effects of a predator. Proc. Natl Acad. Sci. USA98, 3904–3908 (2001). ArticleCASGoogle Scholar
- Trussell, G. C., Ewanchuk, P. J. & Matassa, C. M. Habitat effects on the relative importance of trait- and density-mediated indirect interactions. Ecol. Lett.9, 1245–1252 (2006). ArticleGoogle Scholar
- Wootton, J. T. Indirect effects and habitat use in an intertidal community: interaction chains and interaction modifications. Am. Nat.75, 1544–1551 (1993). Google Scholar
- Vandermeer, J. H. The competitive structure of communities: an experimental approach with protozoa. Ecology50, 362–371 (1969). ArticleGoogle Scholar
- Bairey, E., Kelsic, E. D. & Kishony, R. High-order species interactions shape ecosystem diversity. Nat. Commun.7, 12285 (2016). ArticleCASGoogle Scholar
- Godoy, O. & Levine, J. M. Phenology effects on invasion success: insights from coupling field experiments to coexistence theory. Ecology95, 726–736 (2014). ArticleGoogle Scholar
- Goldberg, D. E. & Werner, P. A. Equivalence of competitors in plant communities: a null hypothesis and a field experiment. Am. J. Bot.170, 1098–1104 (1983). ArticleGoogle Scholar
- Case, T. J. & Bender, E. A. Testing for higher order interactions. Am. Nat.118, 920–929 (1981). ArticleGoogle Scholar
- Anderson, T. L. & Whiteman, H. H. Non-additive effects of intra- and interspecific competition between two larval salamanders. J. Anim. Ecol.84, 765–772 (2015). ArticleGoogle Scholar
- Wilbur, H. M. Competition, predation, and the structure of the Ambystoma–Rana sylvatica community. Ecology53, 3–21 (1972). ArticleGoogle Scholar
- Shmueli, G. To explain or to predict? Stat. Sci.25, 289–310 (2010). ArticleGoogle Scholar
- Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002). Google Scholar
- Adler, P. B., HilleRisLambers, J. & Levine, J. A niche for neutrality. Ecol. Lett.10, 95–104 (2007). ArticleGoogle Scholar
- Okuyama, T. & Holland, J. N. Network structural properties mediate the stability of mutualistic communities. Ecol. Lett.11, 208–216 (2008). ArticleGoogle Scholar
- Novak, M. & Wootton, J. T. Estimating nonlinear interaction strengths: an observation-based method for species-rich food webs. Ecology89, 2083–2089 (2008). ArticleGoogle Scholar
- Abrams, P. A. Implications of dynamically variable traits for identifying, classifying, and measuring direct and indirect effects in ecological communities. Am. Nat.146, 112–134 (1995). ArticleGoogle Scholar
- Dwyer, J. M., Hobbs, R. J., Wainwright, C. E. & Mayfield, M. M. Climate moderates release from nutrient limitation in natural annual plant communities. Global Ecol. Biogeogr.24, 549–561 (2015). ArticleGoogle Scholar
- Law, R. & Watkinson, A. R. Response-surface analysis of two-species competition: an experiment of Phleum arenarium and Vulpia fasciculata . J. Ecol.75, 871–886 (1987). ArticleGoogle Scholar
- Angert, A. L., Huxman, T. E., Chesson, P. & Venable, D. L. Functional tradeoffs determine species coexistence via the storage effect. Proc. Natl Acad. Sci. USA106, 11641–11645 (2009). ArticleCASGoogle Scholar
- Quinn, G. P. & Keough, M. J. Experimental Design and Data Analysis for Biologists (Cambridge Univ. Press, 2002). BookGoogle Scholar
- Rao, C. R., Toutenburg, H. & Shalabh, H. C. Linear Models and Generalizations (Springer, 2008). Google Scholar
- Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S 4th edn (Springer, 2002). BookGoogle Scholar
- Larsen, W. A. & McCleary, S. J. The use of partial residual plots in regression analysis. Technometrics14, 781–790 (1972). ArticleGoogle Scholar
Acknowledgements
This project was made possible by funding awarded to M.M.M. by the Australian Research Council (DP140100574 and FT140100498) and to D.B.S. from the Royal Society of New Zealand (UOC-1101 and a Rutherford Discovery Fellowship). We thank H. R. Lai, X. Loy, C. Wainwright and J. HilleRisLambers for help with data collection and J. HilleRisLambers, J. Dwyer, J. Tylianakis and the Mayfield and Stouffer labs for constructive comments. We also thank X. Loy for the art used to create Supplementary Fig. 1.
Author information
Authors and Affiliations
- The University of Queensland, School of Biological Sciences, Brisbane, 4072, Queensland, Australia Margaret M. Mayfield
- Centre for Integrative Ecology, University of Canterbury, School of Biological Sciences, Christchurch, Canterbury 8041, New Zealand Daniel B. Stouffer
- Margaret M. Mayfield







